We know that getting to the cause of your assessment conditions and implementing appropriate corrective action can be overwhelming!
The good news is that it is possible to conduct cause analysis using best-practice techniques, so you feel confident you have identified the real problem and have addressed it so effectively that it never happens again.
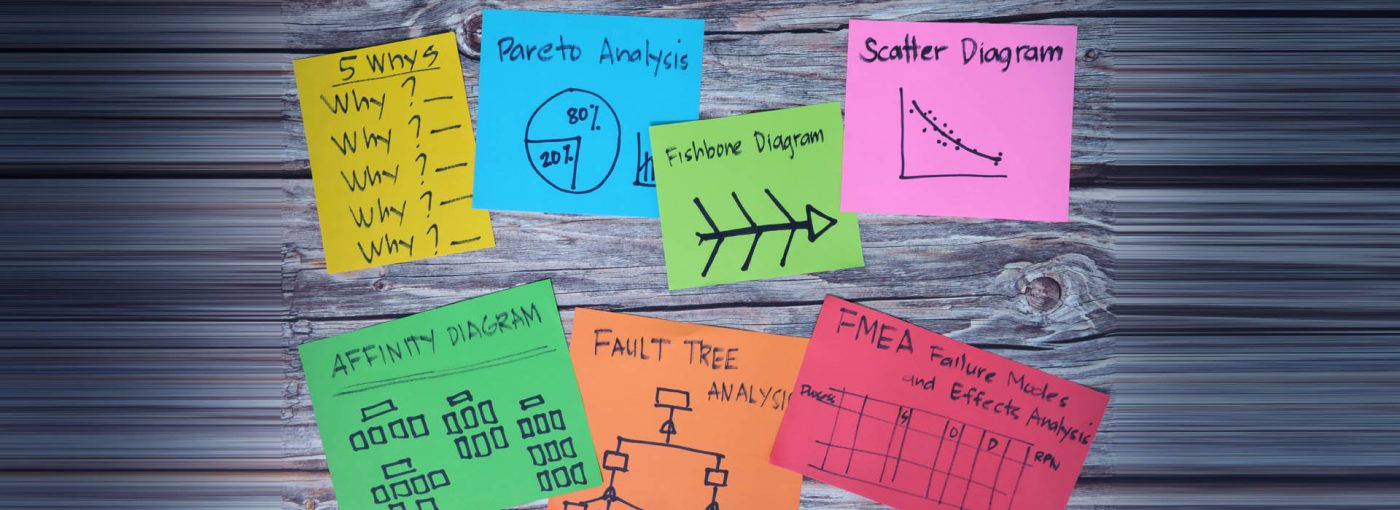
What is cause analysis?
Cause analysis in a testing or calibration laboratory involves systematically identifying the reasons for deviations, errors, or nonconformities in laboratory processes. It focuses on investigating contributing factors, such as equipment malfunctions, procedural flaws or human errors, to pinpoint the cause of the issue rather than addressing only the symptoms.
Why do cause analysis?
Cause analysis is a mandatory part of addressing any conditions raised at a NATA assessment but that’s not the only reason a laboratory might do it.
- Ensuring accuracy and reliability: Cause analysis helps identify and address the causes of errors or deviations, ensuring the reliability of test or calibration results and maintaining confidence in the laboratory’s outputs.
- Conformance with Standards: Accredited laboratories must conform with international standards such as ISO/IEC 17025 and ISO15189, which require effective corrective actions based on thorough cause analysis to maintain accreditation and meet customer expectations.
- Continuous improvement: By identifying underlying issues, cause analysis enables laboratories to refine processes, enhance efficiency and reduce the likelihood of recurring problems, contributing to a culture of continuous quality improvement.
- Risk mitigation: Proactively addressing causes minimises risks associated with faulty results, potential financial losses, reputational damage and legal implications.
- Customer satisfaction: Cause analysis ensures that customer concerns are effectively resolved, fostering trust and long-term relationships with clients.
What is corrective action?
Corrective action in a testing or calibration laboratory refers to the steps taken to eliminate the cause of a nonconformity or error, to prevent its recurrence, and ensure consistent and valid results. It involves identifying the issue through cause analysis, implementing measures to address it, and monitoring the effectiveness of these actions over time. Corrective actions are critical for maintaining conformance with Standards, such as ISO/IEC 17025 and ISO 15189, for NATA accreditation, improving operational quality and building customer trust.
What do laboratories find challenging about cause analysis and corrective action?
NATA finds that laboratories can experience the following challenges when they are completing their cause analysis, which impacts their ability to implement appropriate and effective corrective action.
- Pinpointing the cause: Distinguishing the true cause from contributing factors or symptoms can be complex, especially in cases involving multiple variables or systemic issues.
- Resource limitations: Limited time, staff or expertise can make it difficult to conduct thorough investigations and implement effective corrective actions, particularly in smaller labs.
- Data gaps: Incomplete, inaccurate or poorly documented records can hinder the analysis process, making it challenging to trace issues back to their origin or verify the effectiveness of corrective measures.
- Resistance to change: Staff may be reluctant to acknowledge errors, adapt processes or accept new procedures, especially if the corrective actions disrupt established workflows or require additional effort.
- Lack of systematic tools or training: Laboratories may struggle to execute these processes effectively and consistently without standardised tools or adequate training in cause analysis and corrective action methodologies.
- Complex interconnected processes: Modern laboratory systems are often highly interconnected making it challenging to isolate and address specific issues without impacting other areas.
- Verification of effectiveness: Ensuring that corrective actions have effectively resolved the cause and do not introduce new problems can be a time-consuming and iterative process.
- Maintaining compliance: Laboratories must ensure their approach aligns with relevant Standards (e.g. ISO/IEC 17025 and ISO 15189), which can add complexity and require additional documentation and validation efforts.
Introducing NATA Education’s new Cause Analysis & Corrective Action course
NATA Education now offers a new 1-day course that explores best practice approaches to cause analysis and corrective action to effectively manage risk, quality and non-conformance in the laboratory .
The course is designed to develop a comprehensive understanding of cause analysis and corrective actions and provide an opportunity to practice applying various strategies, techniques and tools to these activities.
Click here to read the full outline and book your place.